Introduction:
Hemophilia is a bleeding disorder characterized by ineffective clot formation due to insufficient thrombin generation. The burden of disease for individuals with hemophilia is high, and less invasive treatment approaches are needed (Machin and Ragni. J Blood Med. 2018). Fitusiran is a once a month subcutaneously administered investigational RNA interference therapeutic targeting antithrombin as a means to improve thrombin generation and promote hemostasis in people with hemophilia A or B, with or without inhibitors. A completed Phase I study demonstrated that monthly subcutaneous administration of fitusiran was generally well tolerated and lowered antithrombin in a dose-dependent manner, resulting in increased thrombin generation and decreased bleeding frequency (Pasi et al. New Engl J Med. 2017). The aim of this abstract is to describe long-term durability, safety and efficacy of monthly fitusiran treatment for patients with hemophilia A or B, with or without inhibitors, in the Phase II open-label extension study.
Methods:
The fitusiran Phase I study (NCT02035605) followed by the Phase II open-label extension study (NCT02554773) included male patients, >18 years of age, with moderate or severe hemophilia A and B, with or without inhibitors. Patients received monthly fixed doses of fitusiran 50 mg or 80 mg subcutaneously. Exploratory post-hoc analysis of bleed events was used to calculate median annualized bleed rate in patients with hemophilia A and B, with or without inhibitors.
Results:
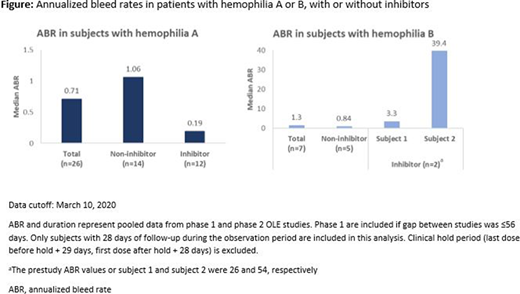
Thirty-four patients (hemophilia A, n=27 [13 with inhibitors and 14 without inhibitors]; hemophilia B, n=7 [2 with inhibitors and 5 without inhibitors) were enrolled in the Phase 2 open-label extension study, and were treated for up to 4.7 years with a median exposure of approximately 2.6 years (as of March 10, 2020). Once-monthly subcutaneous dosing of fitusiran prophylaxis lowered antithrombin (a reduction of between 85% to 72% from baseline) across patients over a sustained period of time. An exploratory analysis of bleeding events showed an overall median annualized bleed rate of 0.84 during the observation period (see figure). Breakthrough bleeds were managed successfully in accordance with the revised bleed management guidelines for reduced doses of bypassing agents and replacement factors. As of March 10, 2020, fitusiran was generally well tolerated and no anti-drug antibody formation was detected.
Conclusions:
Monthly fitusiran prophylaxis provided sustained antithrombin lowering in people with hemophilia A and B, with or without inhibitors, resulting in a low annualized bleeding rate over a median of 2.6 years in an open-label extension study.
Pipe:Medical and Scientific Advisory Council to the National Hemophilia Foundation; Medical Advisory Board to World Federation of Hemophilia: Membership on an entity's Board of Directors or advisory committees; Apcintex, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, F. Hoffmann-La Roche Ltd/Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, uniQure: Consultancy; Siemens: Research Funding. Pasi:Catalyst Biosciences: Consultancy, Other: Personal fees and nonfinancial support; honoraria as member of scientific advisory boards and symposia; Biotest: Consultancy, Honoraria, Other: Personal fees and nonfinancial support; honoraria as member of scientific advisory boards and symposia; Alnylam (Sanofi): Other: Personal fees and nonfinancial support ; Octapharma: Honoraria, Other: Personal fees and nonfinancial support; honoraria as member of scientific advisory boards and symposia , Speakers Bureau; Pfizer: Other; Novo Nordisk: Honoraria, Other: Personal fees and nonfinancial support; honoraria as member of scientific advisory boards and symposia ; Roche: Honoraria, Other; Sobi: Consultancy, Honoraria, Other; Tremeau: Research Funding; Sigilon: Research Funding; ApcinteX: Consultancy, Other: Personal fees ; uniQure: Other: Grants and nonfinancial support , Research Funding; BioMarin: Consultancy, Honoraria, Other: Grants, personal fees, and nonfinancial support; honoraria as member of scientific advisory boards and symposia; Sanofi: Honoraria, Other: Personal fees and nonfinancial support; honoraria as member of scientific advisory boards and symposia, Research Funding; Takeda: Consultancy, Honoraria, Other: Personal fees; honoraria as member of scientific advisory boards and symposia . Lissitchkov:CSL Behring: Other: Principal investigator of clinical trials ; Bayer: Other: Principal investigator of clinical trials ; Novo Nordisk: Other: Principal investigator of clinical trials ; Octapharma: Other: Principal investigator of clinical trials ; Sanofi: Other: Principal investigator of clinical trials ; Roche: Membership on an entity's Board of Directors or advisory committees, Other: lecturer; Shire: Other: lecturer; Sobi: Membership on an entity's Board of Directors or advisory committees, Other: lecturer; Catalyst Biosciences: Other: Principal investigator of clinical trials . Ragni:Alnylam Pharmaceuticals Inc., Baxalta/Takeda, BioMarin, Bioverativ, and Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sangamo: Consultancy, Research Funding; Takeda: Research Funding; Bioverativ: Consultancy, Research Funding; Spark: Consultancy, Research Funding; BioMarin: Consultancy, Research Funding; Alnylam/Sanofi, ATHN, BioMarin, Bioverativ, Sangamo, Spark: Research Funding; Alnylam/Sanofi, BioMarin, Bioverativ, Spark: Consultancy; American Thrombosis Hemostasis Network: Other: Committee work; Baxalta/Takeda, CSL Behring, Genentech, a member of the Roche Group, OPKO Biologics, and Vascular Medicine Institute: Research Funding. Négrier:CSL, F. Hoffmann-La Roche Ltd, Sobi: Other: Travel support; CSL Behring, Octapharma, Shire/Takeda, Sobi: Research Funding; Bayer, Biomarin, CSL Behring, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd, Sanofi, Shire/Takeda, Sobi, Spark: Consultancy. Yu:Sanofi: Other: was an employee and stockholder of Sanofi, at the time of study; Albireo Pharmaceuticals, Inc: Current Employment. Poloskey:Sanofi: Current Employment. Mei:Sanofi: Current Employment, Current equity holder in publicly-traded company. Andersson:Sanofi: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.